This article is descriptive in nature and may be relevant for any brand of car.
Through an exhaust pipe connected to the outlet of the exhaust manifold or manifold head, the exhaust gases are directed to the catalytic converter, and then to the muffler. If the V-shaped engine is equipped with a single exhaust system, the collection of exhaust gases from the two exhaust manifolds into a common exhaust pipe is carried out through the Y-shaped transition provided in it. In vehicles with a dual exhaust system, each exhaust manifold has a separate, independent exhaust system. In most cases, the exhaust pipe consists of several parts so that it can be mounted in the space available under the car. A catalytic converter is installed between the exhaust manifold and muffler (afterburner) in order to reduce the toxicity of exhaust gases. The neutralizer is a casing made of heat-resistant metal (pic. 6.35), which contains a filling of granules coated with a catalyst layer, or a monolithic honeycomb lattice coated with a catalyst layer.
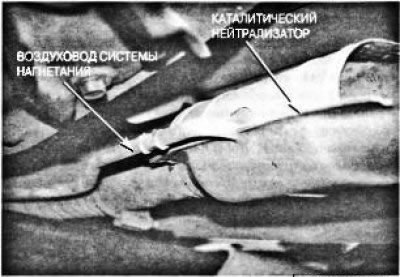
Pic. 6.35. Typical catalytic converter
A thin pipe connected to the side of its body is an air duct coming from the pump of the air injection system. The additional air supplied by the air pump is needed to oxidize toxic compounds and convert them into harmless H20 (water) and CO (carbon dioxide).
The principle of operation of the catalytic converter
The converter uses small amounts of rhodium, palladium and platinum. These chemicals act as a catalyst (a substance that stimulates a chemical reaction but does not itself participate in it). When exhaust gases pass through the catalytic converter, nitrogen oxides are chemically decomposed in its first chamber (NOx) for oxygen and nitrogen. In the second chamber of the catalytic converter, most of the hydrocarbons and carbon monoxide remaining in the exhaust gases are oxidized, resulting in harmless carbon dioxide (CO2) and water vapor (H2O). Some engine designs have a continuous or pulsed air injection system to provide additional air that may be required during the oxidation process (pic. 6.36). In the early 1960s, many converters also used cerium, an element that can store oxygen. The purpose of cerium is to provide oxygen to the catalyst in the event that the exhaust gas mixture is rich and oxygen is insufficient for the complete oxidation of chemical compounds. When the exhaust gas mixture is lean, cerium absorbs excess oxygen.-

Pic. 6.36. Sectional view of a three-way catalytic converter
The air injection pipe is visible, installed in the center between the reduction and oxidation chambers of the neutralizer. Pay attention to the small holes in this pipe, designed to evenly spray the air pumped in by the air pump along the end of the rear, oxidizing, neutralizer chamber.
For the correct operation of the catalytic converter, it is necessary to ensure that the composition of the exhaust gas mixture during its passage through the catalytic converter changes from rich to lean:
- To restore oxygen (ABOUT) from nitrogen oxides (NOx) the mixture should be enriched.
- In order to have enough oxygen to oxidize hydrocarbons (NS) and carbon monoxide (SO) (the reaction of the combination of oxygen with hydrocarbons and carbon monoxide, as a result of which water H2O and carbon dioxide COJ are formed, the mixture must be depleted.
In the event of a malfunction of the catalytic converter, it is necessary to check the correctness of the composition of the fuel-air mixture entering the engine and the serviceability of the ignition system.
Tap test
This is an easy way to check is as follows: knock (easily!) on the catalytic converter housing with a light hammer with a rubber head. If the catalyst substrate has collapsed, then it will make a rattling sound when tapped. If the converter rumbles, then it must be replaced (pic. 6.37). Chapter 8 provides a detailed description of the methodology for testing the exhaust system for capacity
Can a catalytic converter fail, but not because it is clogged with soot?
Yes maybe. Catalytic converters fail not only due to mechanical clogging, but also due to chemical damage, or poisoning. Therefore, the catalytic converter must be checked for more than just physical damage (blockage) by measuring backpressure or vacuum and by tapping, but also by temperature rise. This test, usually performed with an IR pyrometer or propane test, evaluates the performance of the converter.
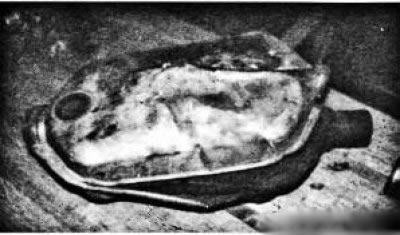
Pic. 6.37. This catalytic converter ruptured as a result of an explosion in it of gasoline contained in a re-enriched exhaust gas mixture. Obviously, clean gasoline got into the catalytic converter and a spark was enough to make it explode. There is no longer any need to diagnose this neutralizer.
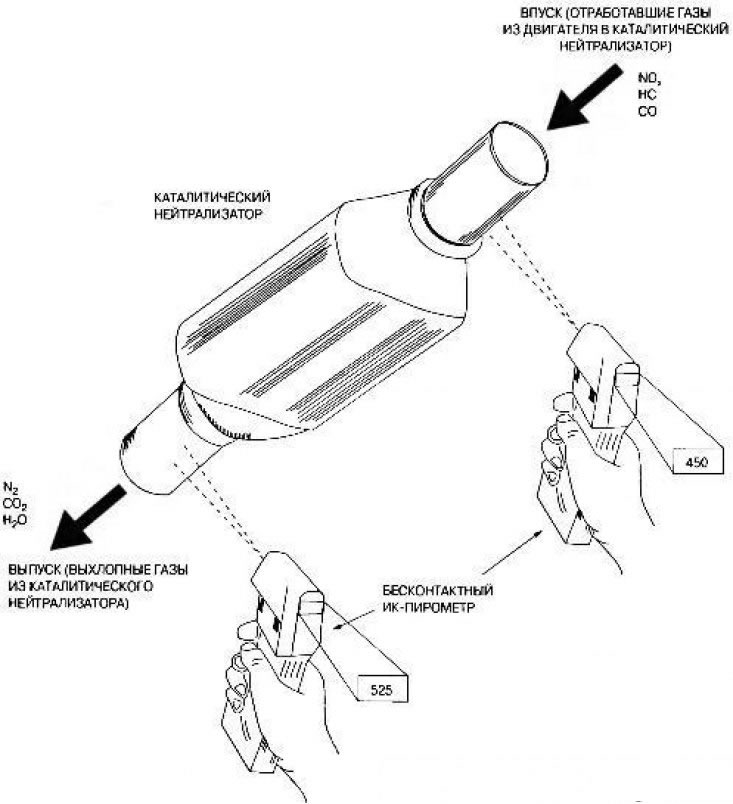
Pic. 6.38. The temperature at the outlet of the converter must exceed the temperature at the inlet to it by at least 10%
This converter is extremely efficient. Its inlet temperature is 450°F (232°С). Ten percent of 450°F is 45°F (450°F+45°F=495°F (257°С)). In other words, in order for the neutralizer to be considered normally functioning, the temperature at its outlet must be at least 495°F. In this case it is 525°F (274°С) and exceeds the temperature at the inlet of the converter by more than 10%. If the neutralizer is generally inoperable, then the temperature at its outlet will be lower than the temperature at its inlet.
Catalytic converters are not "are dying" by themselves.
Catalytic converters stimulate chemical reactions but do not initiate them themselves. Thus. they are not subject to wear and tear. If it is determined that the catalytic converter has failed (out of order or completely clogged), look for the reason why it happened. Remember the following:
"Catalytic converters do not die on their own - their death is always caused by some external cause".
If a catalytic converter failure is detected, all components of the ignition system and fuel system must be included among the tested components. Too much unburned fuel in the exhaust gases can cause overheating and failure of the catalytic converter. To ensure maximum efficiency of the catalytic converter, the correct composition of the fuel-air mixture must be maintained, and for this, the oxygen sensor must be operational and measure the oxygen content at a frequency of 0.5 to 5 Hz.
